| Active Ingredient | AVATROMBOPAG MALEATE |
|---|
| Drug Name | FDA Application No. | Company | Dosage Form;Route | Strength | RLD Strength | Original Approval or Tentative Approval Date |
Exclusivity Expiration (NCE) |
Exclusivity Expiration (ODE) |
Chemical Type |
Review Classification |
Marketing Status |
TE Code |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DOPTELET | 210238 | AKARX INC | TABLET;ORAL | EQ 20MG BASE | EQ 20MG BASE | May 21, 2018 | May 21, 2023 | _ | Type 1 - New Molecular Entity | PRIORITY; Orphan | Prescription | None |
| Parameters | Details |
|---|---|
| Structural Formula |
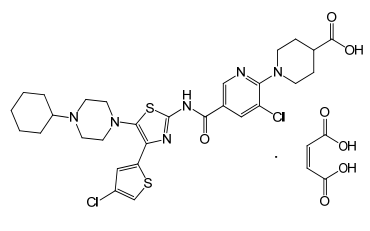 |
| Chemical Name | 4-piperidinecarboxylic acid, 1-[3-chloro-5-[[[4-(4-chloro-2thienyl)-5-(4-cyclohexyl-1-piperazinyl)-2-thiazolyl]amino]carbonyl]-2-pyridinyl]-, (2Z)-2-butenedioate (1:1) |
| CAS No | 677007-74-8 |
| Molecular Formula | C29H34Cl2N6O3S2 · C4H4O4 |
| Molecular Weight | 765.73 |
| Appearance | - |
| Solubility | The aqueous solubility of avatrombopag maleate at various pH levels indicates that the drug substance is practically insoluble at pH 1 to 11 |
| Water Solubility | - |
| Polymorphism | - |
| pKa (Strongest Acidic) | 3.5 |
| pKa (Strongest Basic) | 8.4 |
| Log P | 5.97 |
| Identification | - |
| Degradation | - |
| Hygroscopic | - |
| Photostability study | - |
| Melting Point | - |
| BCS Class | - |
| Manufacture of API | - |
| Parameters | Details |
|---|---|
| Indications and Usage | DOPTELET is a thrombopoietin receptor agonist indicated for the treatment of: • Thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. • Thrombocytopenia in adult patients with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment. |
| Dosage and Administration |
• Administer DOPTELET with food. • Chronic Liver Disease: Dose DOPTELET based upon platelet count prior to procedure, orally for 5 days beginning 10 to 13 days before procedure. For platelet count less than 40 x109/L, the dose is 60 mg (3tablets) once daily; for platelet count 40 to less than 50 x109/L the dose is 40 mg (2 tablets) once daily. • Chronic Immune Thrombocytopenia: Initiate DOPTELET at 20 mg (1 tablet) once daily. Adjust the dose or frequency of dosing to maintain platelet count greater than or equal to 50 x109 /L. Do not exceed 40 mg per day. |
| Mechanism of action | Avatrombopag is an orally bioavailable, small molecule TPO receptor agonist that stimulates proliferation and differentiation of megakaryocytes from bone marrow progenitor cells resulting in an increased production of platelets. Avatrombopag does not compete with TPO for binding to the TPO receptor and has an additive effect with TPO on platelet production. |
| Absorption |
Avatrombopag demonstrated dose-proportional pharmacokinetics after single doses from 10 mg (0.25-times the lowest approved dosage) to 80 mg (1.3-times the highest recommended dosage). Healthy subjects administered 40 mg of avatrombopag had a geometric mean (%CV) maximal concentration (Cmax) of 166 (84%) ng/mL and area under the time-concentration curve extrapolated to infinity (AUC0-inf) of 4198 (83%) ng.hr/mL. The pharmacokinetics of avatrombopag were similar in both healthy subjects and the chronic liver disease population. Absorption The median time to maximal concentration (Tmax) occurred at 5 to 6 hours post-dose. |
| Food Effect | Avatrombopag AUC0-inf and Cmax were not affected when DOPTELET was co-administered with a low-fat meal (500 calories, 3 g fat, 15 g proteins, and 108 g carbohydrates) or a high-fat meal (918 calories, 59 g fat, 39 g proteins, and 59 g carbohydrates). The variability of avatrombopag exposure was reduced by 40% to 60% with food. The Tmax of avatrombopag was delayed by 0 to 2 hours when DOPTELET was administered with a lowfat or high-fat meal (median Tmax range 5 to 8 hours) compared to the fasted state. |
| Distribution | Avatrombopag has an estimated mean volume of distribution (%CV) of 180 L (25%). Avatrombopag is greater than 96% bound to human plasma proteins. |
| Metabolism | Avatrombopag is primarily metabolized by cytochrome P450 (CYP) 2C9 and CYP3A4. |
| Elimination |
The mean plasma elimination half-life (%CV) of avatrombopag is approximately 19 hours (19%). The mean (%CV) of the clearance of avatrombopag is estimated to be 6.9 L/hr (29%). Excretion Fecal excretion accounted for 88% of the administered dose, with 34% of the dose excreted as unchanged avatrombopag. Only 6% of the administered dose was found in urine. |
| Peak plasma time (Tmax) | 5 to 6 hours |
| Half life | 19 hours (19%) |
| Bioavailability | 66-69% |
| Age, gender |
Age (18-86 years), body weight (39-175 kg), sex, race [Whites, African Americans, and East Asians (i.e.,Japanese, Chinese and Koreans)], and any hepatic impairment (Child-Turcotte-Pugh (CTP) grade A, B, and C,or Model for End-Stage Liver Disease (MELD) score 4-23) and mild to moderate renal impairment (CLcr ≥30 mL/min) did not have clinically meaningful effects on the pharmacokinetics of avatrombopag.The effect of age (< 18 years) and severe renal impairment (CLcr < 30 mL/min, Cockcroft-Gault) including patients requiring hemodialysis on avatrombopag pharmacokinetics is unknown. |
| DMF | Status | Type | Submit Date | Holder |
|---|---|---|---|---|
| Not Available | ||||
| Parameters | Details |
|---|---|
| Strength | 20 MG |
| Excipients used | Lactose monohydrate,colloidal silicon dioxide, crospovidone, magnesium stearate and microcrystalline cellulose |
| Composition of coating material | Polyvinyl alcohol, talc, polyethylene glycol, titanium dioxide and ferric oxide yellow |
| Composition of caspule shell | - |
| Pharmaceutical Development | Each DOPTELET tablet contains 20 mg avatrombopag (equivalent to 23.6 mg of avatrombopag maleate) |
| Manufacture of the product | Updated soon.. |
| Tablet / Capsule Image |

|
| Appearance | Round, biconvex, yellow, film-coated tablets debossed with “AVA” on one side and “20” on the other side. |
| Imprint code / Engraving / Debossment | Debossed with “AVA” on one side and “20” on the other side |
| Score | No score |
| Color | Yellow |
| Shape | Round |
| Dimension | 8 mm |
| Mfg by | Kawashima Plant, Eisai Co., Ltd of Japan |
| Mfg for | AkaRx, Inc., Durham, North Carolina 27707 |
| Marketed by | Dova Pharmaceuticals, Inc., Durham, North Carolina 27707 |
| Distributed by | - |
| Application No. | Prod No | Patent No | Patent Expiration | Drug Substance Claim | Drug Product Claim | Patent Use Code | Delist Requested | Link |
|---|---|---|---|---|---|---|---|---|
| N210238 | 1 | 7638536 | May 5, 2025 | DS | DP | - | - | Download |
| N210238 | 1 | 8765764 | January 15, 2023 | - | - | U-2314 U-2578 | - | Download |
| N210238 | 1 | 8338429 | June 30, 2023 | - | - | U-2577 | - | Download |
| USP Apparatus | Speed (RPMs) | Medium | Volume (mL) | Recommended Sampling Times (minutes) | Date Updated |
|---|---|---|---|---|---|
| II (Paddle) | 50 | 0.05M phosphate buffer | 900 mL | Q point at 45 min | As per SBOA |
| Label | Link |
|---|---|
| FDA label | Download |
| FDA chemistry review | Download |
| FDA Pharmacology Review(s) | Download |
| FDA Clinical Pharmacology Biopharmaceutics Review(s) | |
| FDA BE Recommendation | |
| European Public Assessment Report |
| - |
| www.accessdata.fda.gov, www.drugbank.ca, www.ema.europa.eu, www.medicines.org.uk, dailymed.nlm.nih.gov |
